1 PHYSICAL QUANTITIES AND UNITS: Difference between revisions
(Added 1.3.2) |
|||
| Line 55: | Line 55: | ||
{| style="margin: auto;border: 0;width: 100%;" | {| style="margin: auto;border: 0;width: 100%;" | ||
|- | |- | ||
| style="font-style: italic;width: | | style="font-style: italic;width: 100px;" | Examples || $\Delta_{\rm{f}}S^{⦵} (\ce{HgCl2},\ \rm{cr},\ 25 ^{\circ}C) = −154.3\ \rm{J\ K}^{−1}\ \rm{mol}^{−1}$ | ||
|- | |- | ||
| || $\mu_i = (\partial G/{\partial}n_i)_{T,p,\ldots,n_j,\ldots;\ j\neq i}$ or $μ_i = (\partial G/{\partial}n_i)_{T,p,n_{j\neq i}}$ | | || $\mu_i = (\partial G/{\partial}n_i)_{T,p,\ldots,n_j,\ldots;\ j\neq i}$ or $μ_i = (\partial G/{\partial}n_i)_{T,p,n_{j\neq i}}$ | ||
| Line 61: | Line 61: | ||
Vectors and matrices may be printed in bold-face italic type, e.g. $\boldsymbol{A}$, $\boldsymbol{a}$. Tensors may be printed in bold-face italic sans serif type, e.g. <span style="font-family: Verdana, Geneva, Tahoma, sans-serif;font-weight: bold;font-style: italic;">S, T</span>. Vectors may alternatively be characterized by an arrow, $\overrightarrow{A}$, $\overrightarrow{a}$ and second-rank tensors by a double arrow, $\overset{\rightrightarrows}{S}$, $\overset{\rightrightarrows}{T}$. | Vectors and matrices may be printed in bold-face italic type, e.g. $\boldsymbol{A}$, $\boldsymbol{a}$. Tensors may be printed in bold-face italic sans serif type, e.g. <span style="font-family: Verdana, Geneva, Tahoma, sans-serif;font-weight: bold;font-style: italic;">S, T</span>. Vectors may alternatively be characterized by an arrow, $\overrightarrow{A}$, $\overrightarrow{a}$ and second-rank tensors by a double arrow, $\overset{\rightrightarrows}{S}$, $\overset{\rightrightarrows}{T}$. | ||
===1.3.2 General rules for symbols for units=== | |||
Symbols for units should be printed in roman (upright) type. They should remain unaltered in the plural, and should not be followed by a full stop except at the end of a sentence. | |||
{| style="margin: auto;border: 0;width: 100%;" | |||
| style="font-style: italic;width: 100px;" | Example || ${\rm{r}} = 10\ {\rm{cm}}$, not cm. or cms. | |||
|} | |||
When a quantity symbol is used as a symbol for a unit, it is written following the general rules for symbols for quantities (see Section 1.3.1, p. 3). | |||
{| style="margin: auto;border: 0;width: 100%;" | |||
| style="font-style: italic;width: 100px;" | Example || ${\rm{r}} = 1a_0$, where $a_0$ is the bohr, the atomic unit of length. | |||
|} | |||
Symbols for units shall be printed in lower case letters, unless they are derived from a personal name when they shall begin with a capital letter. An exception is the symbol for the litre which may be either $\rm{L}$ or $\rm{l}$, i.e. either capital or lower case (see footnote 2). | |||
{| style="margin: auto;border: 0;width: 100%;" | |||
| style="font-style: italic;width: 100px;" | Examples || $\rm{m}$ (metre), $\rm{s}$ (second), but $\rm{J}$ (joule), $\rm{Hz}$ (hertz) | |||
Decimal multiples and submultiples of units may be indicated by the use of prefixes as defined in Section 2.5, p. 14. | |||
{| style="margin: auto;border: 0;width: 100%;" | |||
| style="font-style: italic;width: 100px;" | Examples || $\rm{nm}$ (nanometre), $\rm{MHz} (megahertz)$, $\rm{kV}$ (kilovolt) | |||
Revision as of 11:34, 15 May 2024
1.1 PHYSICAL QUANTITIES AND QUANTITY CALCULUS
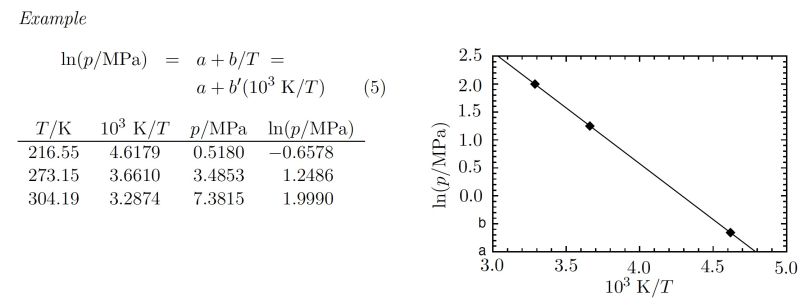
The value of a physical quantity Q can be expressed as the product of a numerical value {Q} and a unit [Q] $Q = \{Q\}[Q] \quad (1)$ Neither the name of the physical quantity, nor the symbol used to denote it, implies a particular choice of unit. Physical quantities, numerical values, and units may all be manipulated by the ordinary rules of algebra. Thus we may write, for example, for the wavelength of one of the yellow sodium lines $\lambda = 5.896\ \times 10^{−7}\ \rm{m} = 589.6\ \rm{nm} \quad (2)$ where m is the symbol for the unit of length called the metre (see Section 2.2, p. 11), nm is the symbol for the nanometre, and the units metre and nanometre are related by $1\ \rm{nm} = 10^{−9}\ \rm{m}\ \rm{or}\ \rm{nm} = 10^{−9}\ \rm{m} \quad (3)$ The equivalence of the two expressions for $\lambda$ in Equation (2) follows at once when we treat the units by the rules of algebra and recognize the identity of 1 nm and $10^{−9}\ \rm{m}$ in Equation (3). The wavelength may equally well be expressed in the form $\lambda/\rm{m} = 5.896\ \times 10^{−7}\ \rm{or}\ \lambda/\rm{nm} = 589.6 \quad (4)$ It can be useful to work with variables that are defined by dividing the quantity by a particular unit. For instance, in tabulating the numerical values of physical quantities or labeling the axes of graphs, it is particularly convenient to use the quotient of a physical quantity and a unit in such a form that the values to be tabulated are numerical values, as in Equations (4).
Algebraically equivalent forms may be used in place of $10^3\ \rm{K/T}$ , such as $\rm{kK}/T$ or $10^3\ (T/\rm{K})^{−1}$. Equations between numerical values depend on the choice of units, whereas equations between quantities have the advantage of being independent of this choice. The method described here for handling physical quantities and their units is known as quantity calculus (see ISO [5.a] and [11–14]). It is recommended for use throughout science and technology. The use of quantity calculus does not imply any particular choice of units (see Section 3.1, p. 15 for quantity calculus).
1.2 BASE QUANTITIES AND DERIVED QUANTITIES
By convention physical quantities are organized in a dimensional system built upon seven base quantities, each of which is regarded as having its own dimension. These base quantities in the International System of Quantities (ISQ) on which the International System of units (SI) is based, and the principal symbols used to denote them and their dimensions are as follows:
- Insert Table**
All other quantities are called derived quantities and are regarded as having dimensions derived algebraically from the seven base quantities by multiplication and division.
Example: dimension of energy is equal to dimension of $M\ L^2\ T^{−2}$. This can be written with the symbol dim for dimension (see footnote 1, below) $\dim(E) = \dim(m·l^2·t^{−2}) = M\ L^2\ T^{−2}$
The quantity amount of substance is of special importance to chemists. Amount of substance is proportional to the number of specified elementary entities of the substance considered. The proportionality factor is the same for all substances; its reciprocal is the Avogadro constant (see Section 4.10, p. 44). The SI unit of amount of substance is the mole. "Amount of substance" is also called “chemical amount”, and may be abbreviated to the single word "amount", particularly in such phrases as “amount concentration” (see footnote 2), and "amount of $\ce{N2}$". In the same sense one might for instance say “amount concentration of $\ce{N2}$.” A possible name for international usage has been suggested: “enplethy” [10] (from Greek, similar to enthalpy and entropy).
In the ISQ, electric current is chosen as base quantity and ampere is the SI base unit. In atomic and molecular physics, the so-called atomic units are useful (see Section 2.4, p. 12).
Ordinal quantities and nominal properties are outside the scope of the Green Book [9].
1.3 SYMBOLS FOR PHYSICAL QUANTITIES AND UNITS [5.a]
A clear distinction should be drawn between the names and symbols for physical quantities, and the names and symbols for units. Names and symbols for many quantities are given in Chapter 4, p. 21; the symbols given there are recommendations. If other symbols are used they should be clearly defined. Names and symbols for units are given in Chapter 2, p. 11; the symbols for units listed there are quoted from the Bureau International des Poids et Mesures (BIPM) and are mandatory.
1.3.1 General rules for symbols for quantities
The symbol for a physical quantity should be a capital or lower case single letter (see footnote 1) of the Latin or Greek alphabet (see Section 1.6, p. 5). The letter should be printed in italic (sloping) type. When necessary the symbol may be modified by subscripts and superscripts of specified meaning. Subscripts and superscripts that are themselves symbols for physical quantities or for numbers should be printed in italic type; other subscripts and superscripts should be printed in roman (upright) type.
| Examples | $C_{\rm{p}}$ | for heat capacity at constant pressure | |
| $p_{\rm{i}}$ | or partial pressure of the i th substance | ||
| but | $C_{\rm{p}}$ | for heat capacity of substance $\ce{B}$ | |
| $\mu_{\rm{B}}^{\alpha}$ | for chemical potential of substance $\ce{B}$ in phase $\alpha$ | ||
| $\Delta_{\rm{r}} H^{⦵}$ | for standard reaction enthalpy |
The meaning of symbols for physical quantities may be further qualified by the use of one or more subscripts, or by information contained in parentheses.
| Examples | $\Delta_{\rm{f}}S^{⦵} (\ce{HgCl2},\ \rm{cr},\ 25 ^{\circ}C) = −154.3\ \rm{J\ K}^{−1}\ \rm{mol}^{−1}$ |
| $\mu_i = (\partial G/{\partial}n_i)_{T,p,\ldots,n_j,\ldots;\ j\neq i}$ or $μ_i = (\partial G/{\partial}n_i)_{T,p,n_{j\neq i}}$ |
Vectors and matrices may be printed in bold-face italic type, e.g. $\boldsymbol{A}$, $\boldsymbol{a}$. Tensors may be printed in bold-face italic sans serif type, e.g. S, T. Vectors may alternatively be characterized by an arrow, $\overrightarrow{A}$, $\overrightarrow{a}$ and second-rank tensors by a double arrow, $\overset{\rightrightarrows}{S}$, $\overset{\rightrightarrows}{T}$.
1.3.2 General rules for symbols for units
Symbols for units should be printed in roman (upright) type. They should remain unaltered in the plural, and should not be followed by a full stop except at the end of a sentence.
| Example | ${\rm{r}} = 10\ {\rm{cm}}$, not cm. or cms. |
When a quantity symbol is used as a symbol for a unit, it is written following the general rules for symbols for quantities (see Section 1.3.1, p. 3).
| Example | ${\rm{r}} = 1a_0$, where $a_0$ is the bohr, the atomic unit of length. |
Symbols for units shall be printed in lower case letters, unless they are derived from a personal name when they shall begin with a capital letter. An exception is the symbol for the litre which may be either $\rm{L}$ or $\rm{l}$, i.e. either capital or lower case (see footnote 2).
| Examples | $\rm{m}$ (metre), $\rm{s}$ (second), but $\rm{J}$ (joule), $\rm{Hz}$ (hertz)
Decimal multiples and submultiples of units may be indicated by the use of prefixes as defined in Section 2.5, p. 14.
|